While I write mostly about fisheries, by far the two most read blog-posts I have written (over 5000 visits!), relate to the levels of mercury in tuna in relationship to the area of capture (here - 2017 and here -2019)… It seems that more people is worried about eating them, than about their sustainability, legality or the welfare of those that catch it… which is saying something!
The latest SPC Fisheries Bulletin, presents an article by some of the authors of that 2019 paper I blogged about that presents their findings in a “less formal” way than a scientific paper and with some amazing illustrations, is totally recomendable (as al of the articles of the SPC fisheries bulletin!) read it from here!
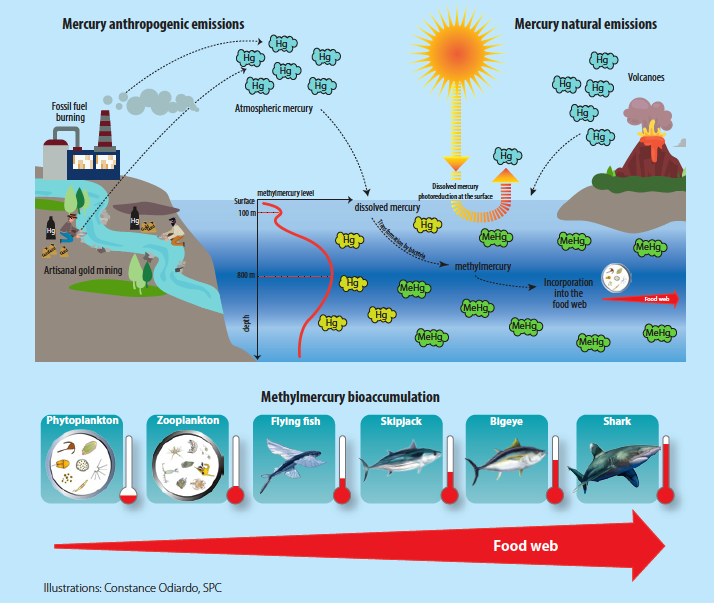
I really like a section that explain where methylmercury in the ocean come from, and the illustration, so I quote it below… yet as usual, read the original!
Mercury is emitted into the atmosphere through natural sources such as volcanoes, but more extensively through human activities such as burning coal and fossil fuels, industrial waste, and small-scale gold mining. The gaseous form of mercury in the atmosphere is gradually deposited into the oceans in the form of dissolved mercury, or methylmercury. Human-caused mercury emissions have been, for example, responsible for a threefold increase in mercury concentrations dissolved in the surface layers of the world’s oceans since the industrial revolution (Lamborg et al. 2014). A fraction of the dissolved mercury is naturally converted into methylmercury by sulphate-reducing bacteria, in which case the process is referred to as mercury methylation. This conversion is particularly intense in the less oxygenated deeper ocean waters (between depths of 400 m and 800 m). Also, in the surface layers, the dissolved methylmercury and mercury are degraded by light and re-emitted into the atmosphere in a gaseous mercury form (photo-reduction process). The production of methylmercury in the oceans, therefore, depends on the balance between methylation, which is more intense in less oxygenated zones (deeper ocean layers), and photo-reduction, which is more intense in surface ocean layers. The balance between these reactions explains the trend towards an increase in methylmercury concentrations with depth.
This methylmercury is highly bioavailable for ingestion and fixing by the living organisms at the base of the food web. Its concentration increases naturally, by accumulation, because it is only eliminated in very small quantities by organisms, from the very first stages in the food web (plankton), up to the top predators (tuna and sharks), which contain the highest methylmercury levels. There are, however, many more grey areas in the ocean mercury cycle.